A lot of effort goes into creating materials that can resist sticking, repel water, or keep oils off of cell phone screens. But some plants are able to accomplish tasks like these naturally. In this post we look at one amazing example…but first, a crash course in surface energy and surface wetting.
Surface energy and wetting
How well a liquid will wet a surface depends on the surface energies of the materials and the surface texture of the substrate.
In terms of material properties, a liquid with a low surface energy (such as gasoline) will readily wet a surface with a high surface energy (such as glass). On the other hand, a liquid on a low energy substrate will wet poorly, beading up like water on Teflon. The chart below shows some common materials and their surface energies.
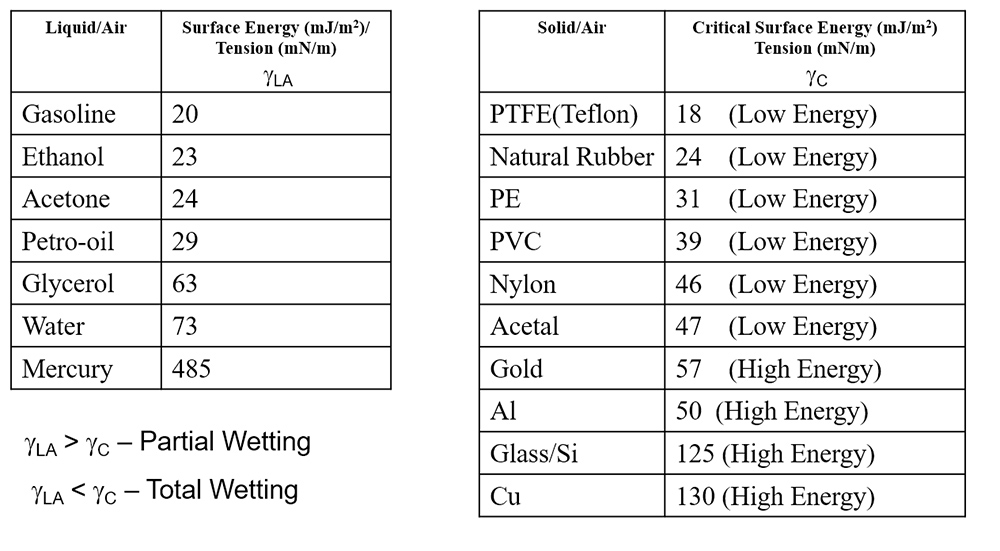
https://en.wikipedia.org/wiki/Surface_energy
Partial wetting occurs when the surface energy of the liquid is high relative to that of the substrate. When the surface energy of the substrate greatly exceeds that of the liquid, total wetting results.
Hydrophobic and Hydrophilic
We refer to a surface as “hydrophobic” if liquids tends to bead up on it rather than wet the surface, as in the second image in the figure below. A liquid on a “hydrophilic” surface, however, tends to wet the surface more readily, as in the top image.
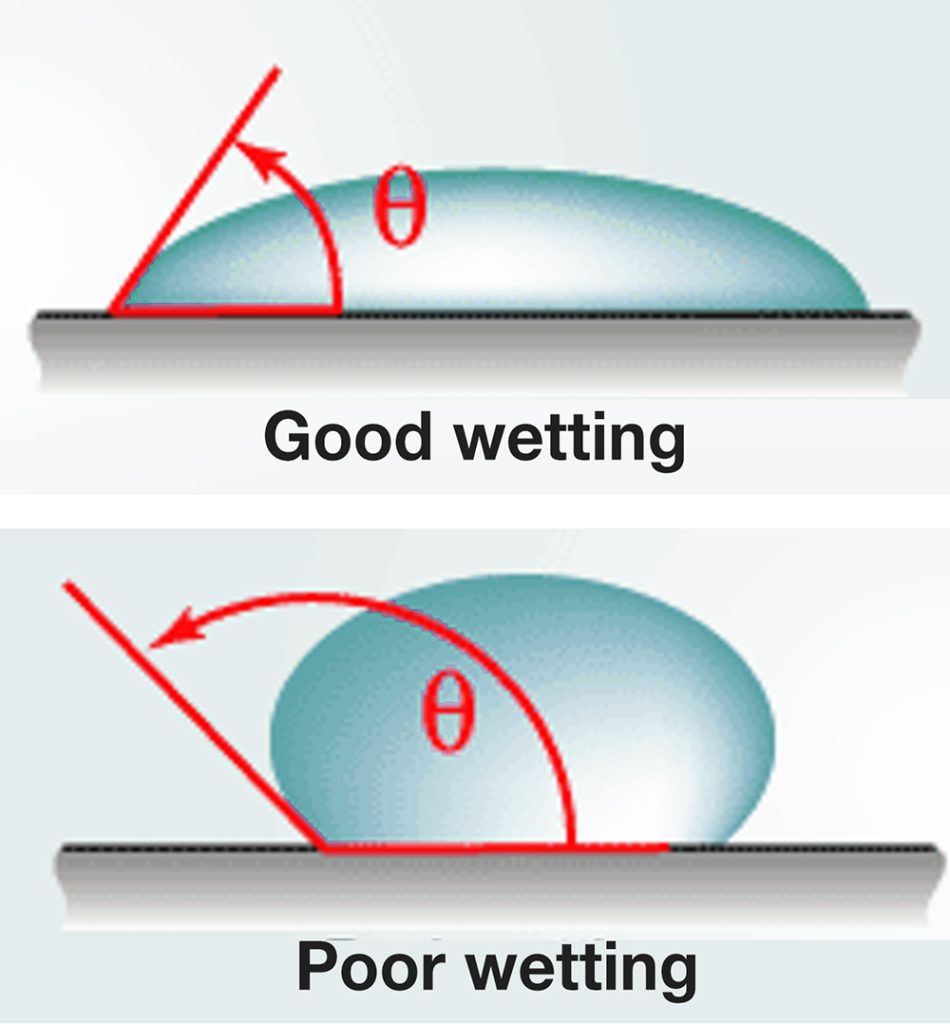
http://web.mit.edu/1.63/www/Lec-notes/Surfacetension/Lecture5.pdf
Surface roughness
But material properties are just part of the story. The surface texture of the substrate will also determine how well a fluid will wet it. Surface roughness always magnifies the underlying wetting properties: greater roughness will make a hydrophobic surface more hydrophobic or a hydrophilic surface more hydrophilic. As you may have anticipated, the spatial wavelengths that make up the texture also play a role in how well the fluid can enter the crevices of the texture versus bridging across the texture.
Enter the lotus
The semi-aquatic lotus plant has the remarkable ability to remain clean even in its muddy environment. By staying clean, the leaves can maximize their use of sunlight to fuel the plant.

This remarkable ability (the “Lotus Effect”) is due, to an extent, to the waxy crystals which coat the upper surface of the leaf and resist wetting.
But the key to making the lotus leaf superhydrophobic is its surface texture. The texture is strong in two wavelengths. Longer wavelength texture leads the water to condense on the leaf surface. Meanwhile, a pattern of small-wavelength, dense structures called papillae cause the condensing water to build up into droplets that eventually roll off of the leaf—taking dirt and bugs with it, maintaining efficient photosynthesis for the plant.
There’s a lot more to be said about surface energy and surface roughness—and we’ve only scratched the surface (!). If you’d like to know more, check out the Surface Energy module of our Surface Roughness, Texture, and Tribology short course, available online at Udemy and in our live course, which is coming up on May 3–5, 2023!
