Artificial medical devices prolong life and preserve quality of living. To design devices that will function reliably and safely for decades requires careful attention to materials and surface finish. Accurately specifying, measuring and controlling surface finish are all of paramount importance.
In this article we explore some examples of how surface texture affects performance of medical devices, and how we can go about controlling texture to maintain longevity and function.
Bearing surfaces
Replacement hips, knees and other joints are designed to last upwards of twenty years, without the benefit of periodic lubrication or resurfacing. In these joints, metal components are bonded to the bones, and these components are separated by (and continuously rub against) a bearing surface.
In most cases the metal surfaces are polished to the highest level of smoothness in order to minimize friction and wear. In the implant shown below, for example, the bearing surface has an Areal Average Roughness (Sa) value of 0.10 microns—a mirror finish. Some implant device may even be held to Angstrom-level average roughness.
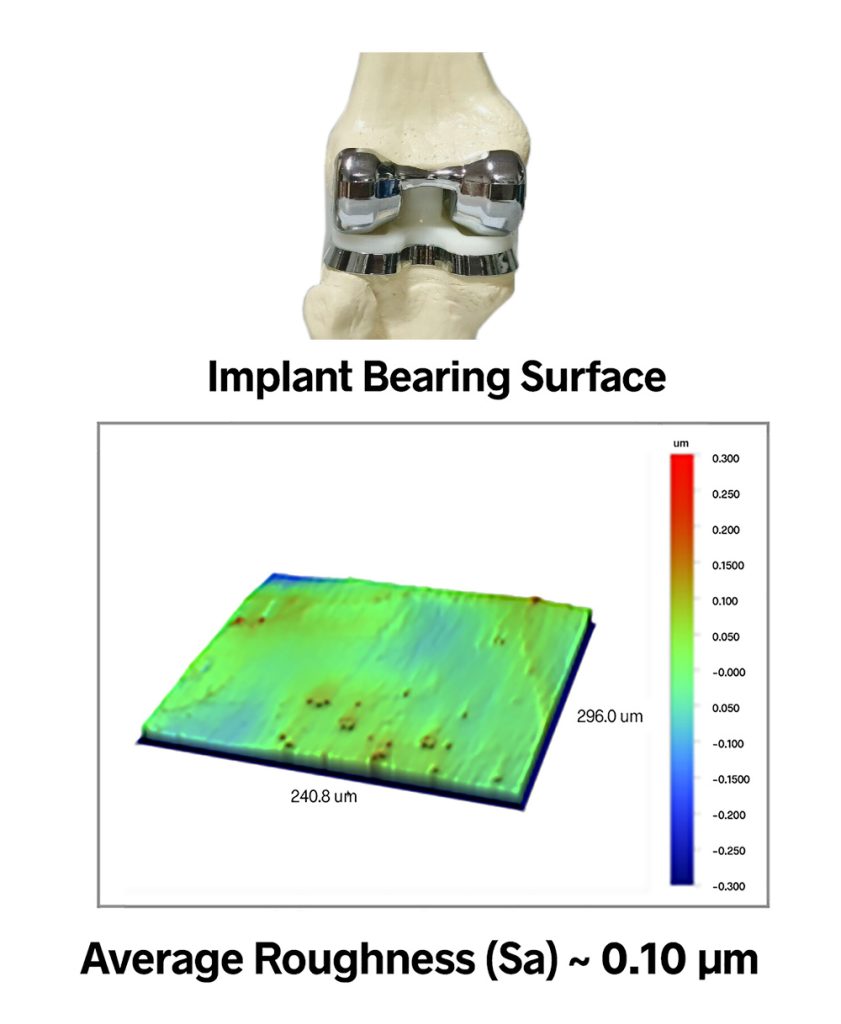
Instinctually, it seems that a polished surfac would provide the least friction and thus would wear best. But this really depends on the application. If there is sufficient synovial fluid in the joint, then the smoothest possible surfaces are indeed best. If components are rubbing directly against each other, however, you need some texture, both to reduce the real area of contact and to retain whatever small amount of lubricant is available.
The nature of the texture also matters. Surface texture parameters such as Mean Summit Curvature (Ssc) and Summit Density (Sds), as well as bearing area parameters and tribology parameters, combine to provide a much clearer prediction of friction and wear than an average roughness value alone.
Another important aspect to remember is that a component manufactured with a mirror finish will not look that way for long. Peaks in the texture will wear quickly during the initial use period. After this wear-in period, the surface will reach a steady state that will, hopefully, change only slowly over the majority of the component’s life. Product development, therefore, must not just focus on making a joint that functions optimally at time-zero, but it must determine the characteristics of an initial finish that will wear to a long-lasting joint after wear-in.
Bonding surfaces
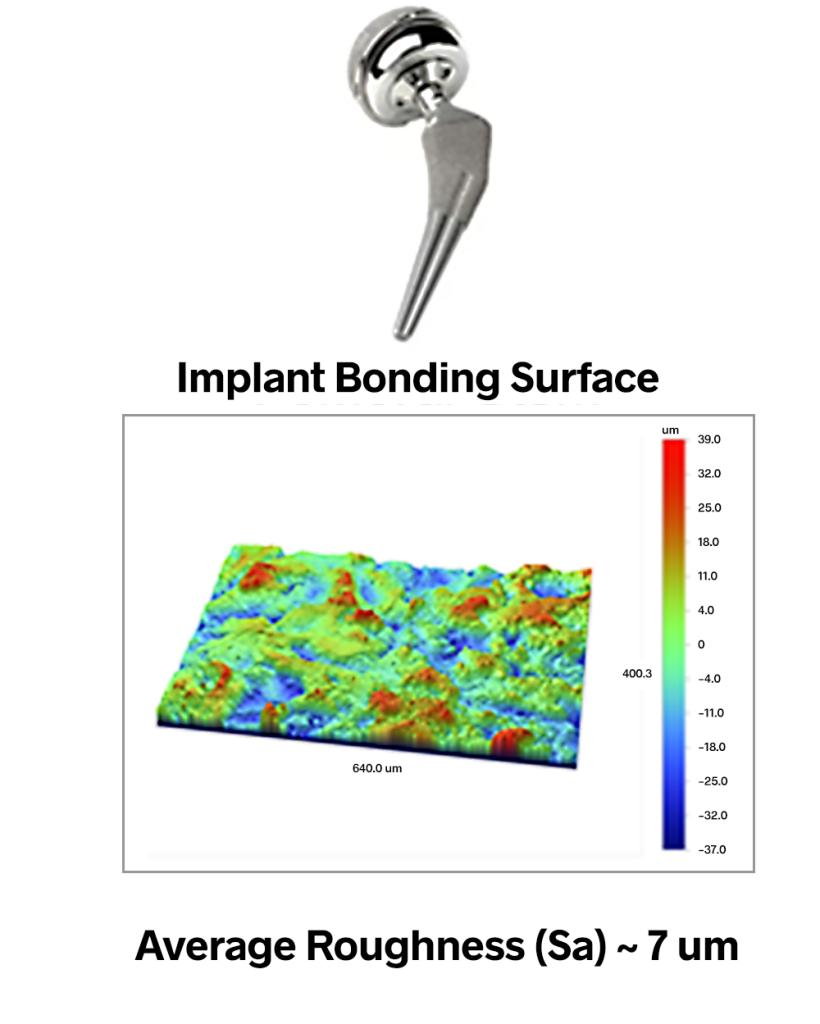
While the mating surfaces of a replacement joint typically need to be very smooth, the bonding areas of these components must instead be very rough in order to provide sufficient surface area for a good bond. In the hip implant shown below, the femoral stem (which bonds to the femur bone) has an average roughness value of approximately 7 microns. These components undergo aggressive processes such as shot blasting to create finishes with Sa values as high as 20 microns—texture that is absolutely visible to the eye.
Again, however, it is not enough to simply “roughen up” the surface: the nature of the texture matters. One typically wants uniform, isotropic texture, as any directionality could lead to slipping or swiveling of the joint. Research has also shown that particular amplitudes and spacings of texture can promote more rapid bone growth. The Sdr (Developed Interfacial Area Ratio) parameter provides information about the texture amplitude and spacing that can lead to better control of adhesion.
Measuring finish on complex surfaces
Complex surface shapes make it very difficult or impossible to measure with many standard instruments. For example, measuring the small, highly curved features of a spinal implant would be extremely challenging with a traditional stylus profiler.
Non-contact, 3D optical profilers are well-suited to these kinds of measurements, however. Their long working distance and large vertical range enable measurement of highly curved surfaces. Fixtures and mirrors give access to otherwise blind features.
Surface finish for flow
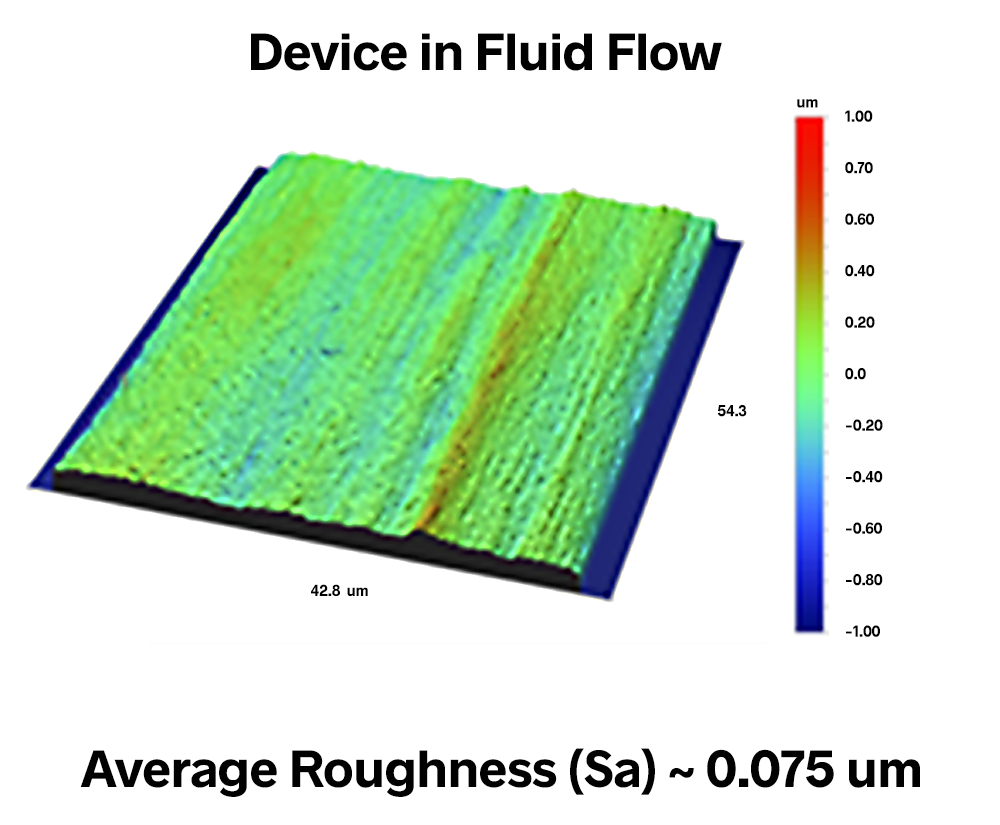
The surface finish for components involved with the flow of bodily fluids must meet a dramatically different set of functional requirements. The surfaces over which the blood flows, for example, must remain extremely smooth throughout their lifetime to prevent clot formation processes.
The scale of the texture that matters in this case, however, may not be the scale of the blood cells. It may be much finer spaced and lower amplitude texture that proteins produced by the cells could interact with to initiate the clotting mechanism.
In devices that contact blood, manufacturers aim for the smoothest possible finishes, often less than 100 nm average roughness (Sa), as in the image below. Smooth finishes also prevent damage to blood cells from processes such as hemolysis as the cells are forced through constrictions.
Michigan Metrology has completed hundreds of projects to address wear and functionality of medical devices. Contact us today to learn how we can help with your particular device design or process challenge.
You may also want to check out our live and online Surface Texture and Wear Classes which offer a wealth of information on the topic.
